When writing my posts on chocolate coated coffee beans and cold brew coffee, I had some hard time finding information on caffeine solubility on the internet. By coffee and tea being a really important part of our society, I thought the information about its basic – and most important – component should be easily available on the internet. Well, think again. Besides same basic descriptions and parameters in Wikipedia (where else), there were surprisingly few information available. Therefore I compiled some data on caffeine solubility, content in coffee beans and extraction efficacy from coffee beans to serve as a background reading for my previous posts. And to serve me as a reference for all my future research.
Caffeine solubility

Water solubility of caffeine is really high and is usually not an issue when preparing your caffeinated drink. It is, as almost all solubilities, temperature dependant. Sadly, it is not that easy to get the solubility data on the internet – wikipedia lists solubility at three different temperatures while further search adds two additional temperatures and their corresponding solubilities.
To get some more points in the construction of the graph, I had to resort to scientific articles, although values found were in the range from 20 to 30 °C, which was relatively narrow for my graph. Nevertheless, it confirmed the caffeine solubility value at 25°C found in Wikipedia – several articles listed comparable values for this temperature.

The results are listed in the table and graph below. As usual for the solubility, the caffeine solubility curve is exponential. The equation is presented on the graph, so one can calculate approximate solubility at any given temperature. The calculated value is obviously only an approximation, but fairly good one in my opinion.
Table 1: Caffeine solubility at differrent temperatures
| Temperature (°C) | Caffeine solubility | Source |
| 0 | 0.6 g/100 mL | Solubilities of inorganic and organic compounds, 2nd ed. |
| 15 | 1.0 g/100 mL | Solubilities of inorganic and organic compounds, 2nd ed. |
| 19.3 | 1.61 g/100 mL | Thermodynamics of caffeine aqueous solutions |
| 25 | 2.17 g/100 mL | Wikipedia |
| 28.9 | 2.54 g/100 mL | Thermodynamics of caffeine aqueous solutions |
| 80 | 18.0 g/100 mL | Wikipedia |
| 100 | 67.0 g/100 mL | Wikipedia |
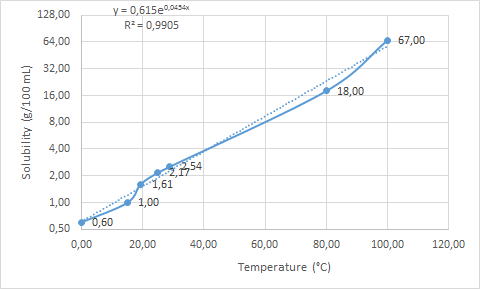
Caffeine solubility at different temperatures
Water solubility of caffeine is of course the main solubility that is interesting, but solubility in other solvents might also prove useful. If you are making ethanolic coffee preparations or just want to do some caffeine extraction, than you should look into that. The table below is a list of some solvents and corresponding solubility at given temperature.
Table 2: Solvents solubility at given temperature
| Solvent | Temperature (°C) | Solubility | Source |
| Ethanol | 60 | 5.0g/100 mL | IARC monograph |
| Acetone | 20 | 2.0g/100 mL | IARC monograph |
| Chloroform | 20 | 18.2g/100 mL | IARC monograph |
| Diethyl ether | 20 | 0.2g/100 mL | IARC monograph |
| Benzene | 20 | 1.0g/100 mL | IARC monograph |
| Acetic acid | 21.5 | 2.4g/100mL | The solubility of acetanilide, phenacetin, caffeine, and salol in several solvents |
| Ethanol | 25 | 0.6g/100 mL | Solubility profiles for the xanthines in aqueous alcoholic mixtures I |
| Methanol | 25 | 1.0g/100 mL | Solubility profiles for the xanthines in aqueous alcoholic mixtures I |
Caffeine content in coffee beans

Caffeine content in coffee beans can, as any material coming from nature, vary depending on many factors. The main difference is the plant source of the beans – beans originating from arabica (Coffea arabica) have around half of the caffeine content than those coming from robusta (Coffea robusta or Coffea canephora). Besides this, time of the year, ripnes of beans when picked, weather conditions, geographic location and so on can also have a significant effect on caffeine content.
But what is the approximate content and how much can it vary?
Well, the standard reference – Wikipedia – puts caffeine content of arabica between 0.8-1.4% and robusta between 1.7-4%. The source is International coffee organization, although the link from Wikipedia reference points at empty location. Other Wikipedia resource claims 2.7% caffeine content in robusta and 1.5% caffeine content in arabica beans and cites this book as reference. The values are not an exact match, again confirming variability in the caffeine content.
Some further research yielded the list of data from scientific literature, which is shown below, together with the sources. The average is around 1% for arabica and 2-3% for robusta. The variations for arabica are from 0.8 to 1.7% and 1.9-4% for robusta, both inline with the Wikipedia data. One can see there is great variability and that the values can differ around twofold. One can use the averages for mixes of coffee bought in retail stores, which by blending even out the variations. Speciality coffee, coming from single region and single produces can, however, have greater variability.
Table 3: Caffeine content in Arabica and Robusta coffee beans
Caffeine extraction

Not just the amount of caffeine and the caffeine solubility in water will determine caffeine content in your coffee. The speed with which the caffeine goes into water is also crucial.
To put in simpler terms – if the caffeine goes slowly from the beans into the coffee, there might not be enough time for all the caffeine to be extracted during a short preparation time. The speed is dependant upon many facts like size of coffee grains, difference in amount of caffeine in grains and in water, temperature, time of extraction and if the whole thing is mixed or not (i.e. mechanical agitation).
Since the caffeine solubility in water is really high, probably all the caffeine is extracted from the beans (i.e. really small amounts are left in the beans), if the extraction time is long enough to allow all the caffeine to pass into water. Scientific literature is confirming my reasoning (here and here). Particle size has a great influence on the speed with which the caffeine is extracted – the smaller the particles, faster the extraction – while mixing did not have an influence at a given particle size. Temperature has even stronger effect – if it was raised from around 20 to 80 (four fold), the extraction speed increased eightfold.
Conclusion
What is needed for strong coffee? The best way to make a strong coffee is to use more coffee beans per cup of coffee. This is by far the most simple and effective way. The amount of caffeine in the beans might vary, but it is hard to determine the exact amount – again, just add more beans per cup. To get all the caffeine out of the beans, just prepare the coffee with hot water, where extraction time is fast, if doing a cold brew – well just prolong your extraction times. However, if you want to be really sure and get 100% utilization of caffeine, just eat the whole beans!









Two of my favorite methods for making coffee are a French press and a Vietnamese phin.
With the phin, the finely ground coffee is exposed to water for about five minutes; traditionally, the water should make one drip per second. An advantage of a phin is the water is carried downward through the ground coffee, being restrained from floating by the pressure plate. The biggest advantage is you can stop the brewing before the bitter, sour coffee comes through. The result is a roughly espresso size portion and coffee/water ratio.
The question is, would most of the caffeine be extracted in five minutes? How much caffeine would be left in the tail end bitter brew?
Brewing notes: I put about two tablespoons of water at about 80 C in the phin, wait 30-60 seconds, then put hotter water in, about 90 C. Some use even hotter water, up to boiling.
I also use a French press, and am particularly happy with the new models with the plastic frame holding a very fine mesh metal filter, plus a silicone gasket on the outer ring. This allows the use of fine ground coffee, if you wish, because none gets through, but the fines.
Since I steep my French press coffee for a total of about 5 minutes, including plunging, and use much more water than a phin, I assume this would extract more caffeine. However, if a French press is used correctly, much of the bitter brew remains in the grounds, or at least at the bottom of the pot.
I presume the French press would extract more caffeine, which is ironic since the phin produces a much stronger tasting coffee, but less of it.
The AeroPress is another matter, and its use is less standardized. However, the pressure does speed flavor extraction. So it would follow that pressure would also speed caffeine extraction.
There are also new travel presses, such as the Kohipress, that are narrow French presses, but that also allow you to create pressure on the steeping grounds. I steep about five minutes, too. They make excellent coffee. Perhaps they extract more caffeine than a regular French press?
It sounds like, all things being equal, the stronger the coffee, the more caffeine is extracted. But, given the temperature graph, the better tasting the coffee, the less caffeine is extracted.
On the other hand, I read that high temperatures break down caffeine. This contradicts the temperature/extraction graph. Does this mainly happen at temperatures above the boiling point of water, and/or in air rather than water?
Just wondering.
Thank you for the questions!
A real brain teaser!
I haven’t studied the extraction kinetics (speed). Definitely will look into in in the future.
As for the break down on the caffeine – 100 C is way too low for any serious degradation